Real-world evidence generation with David Thompson
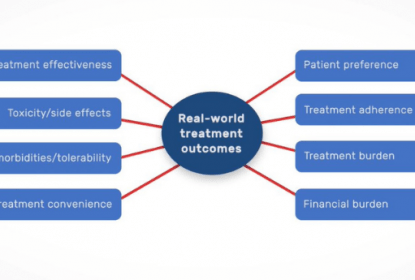
In this video David Thompson, Syneos Health, provides his thoughts on the role of real-world evidence (RWE) in healthcare decision making. Reflecting on recent examples where RWE has been used by regulatory bodies in drug approvals, David also discusses the importance of well-designed RWE studies to ensure it can be considered by regulatory bodies and how new technologies, such as virtual research, are being used to generate RWE.
About the author:
David Thompson, PhD is Senior Vice President, Real World Evidence Advisory at Syneos Health. David is a health economist with 30 years of experience in the health economics arena, including work in economic modeling, retrospective database analysis, trial-based economic evaluations, and patient-reported outcomes. David is the Editor-in-Chief of Value & Outcomes Spotlight, the journal of the Professional Society for Health Economics and Outcomes Research (ISPOR), and a steering committee member of the Clinical Trials Transformation Initiative (CTTI), a multistakeholder initiative of Duke University and the FDA, and a member of the CTTI initiative in real world evidence. David can be reached at [email protected].