Analysis of patients’ side effects and quality of life in the ADAURA study
This animated video article presents results from the phase III ADAURA study investigating osimertinib for the treatment of patients with early-stage resectable epidermal growth factor receptor (EGFR)-mutated non-small cell lung cancer (NSCLC).
Osimertinib is a drug that blocks the activity of a protein called EGFR on cancer cells, reducing their growth and spread, and is the recommended treatment in patients with early-stage or advanced NSCLC with certain types of EGFR mutations.
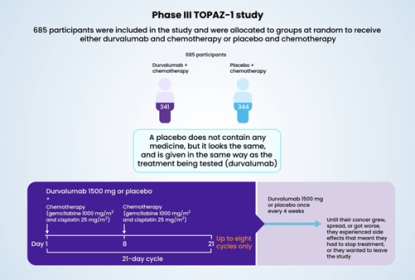
The ADAURA study investigated osimertinib versus placebo after surgery in patients who have early‑stage NSCLC with a mutation in the EGFR gene. Here, we present a summary of the 3-year safety, tolerability and health-related quality of life outcomes from ADAURA.
Patients remained alive and cancer-free for longer with osimertinib compared with placebo, and most of the side effects were mild or moderate. The most common side effects with osimertinib were diarrhoea, nail changes, dry skin and itchy skin and most patients did not need to reduce, pause or stop their treatment due to side effects. The quality of life of most patients remained consistent over the treatment period.
These data show that osimertinib treatment after surgery can prolong the time patients remain alive and cancer-free, side effects were manageable and the patients’ quality of life was maintained.
The content of this animated video article is based on the authors’ manuscript, “Three-Year Safety, Tolerability, and Health-Related Quality of Life Outcomes of Adjuvant Osimertinib in Patients With Resected Stage IB to IIIA EGFR-Mutated NSCLC: Updated Analysis From the Phase 3 ADAURA Trial” published in the Journal of Thoracic Oncology.
Find out more about the original study in our Plain Language Summary of Publication, published in Future Oncology.
Original article:
John T, Grohé C, Goldman JW et al. Three-Year Safety, Tolerability, and Health-Related Quality of Life Outcomes of Adjuvant Osimertinib in Patients With Resected Stage IB to IIIA EGFR-Mutated NSCLC: Updated Analysis From the Phase 3 ADAURA Trial. J Thorac Oncol. 2023;18(9):1209-1221. doi: 10.1016/j.jtho.2023.05.015
Publication DOI: https://doi.org/10.1080/vjbm-2023-0012